Chloralkali process
It is the technology used to produce chlorine and sodium hydroxide (caustic soda),[1] which are commodity chemicals required by industry.
Usually the process is conducted on a brine (an aqueous solution of concentrated NaCl), in which case sodium hydroxide (NaOH), hydrogen, and chlorine result.
The process has a high energy consumption, for example around 2,500 kWh (9,000 MJ) of electricity per tonne of sodium hydroxide produced.
The membrane cell process, which was only developed in the past 60 years, is a superior method with its improved energy efficiency and lack of harmful chemicals.
Although the first formation of chlorine by the electrolysis of brine was attributed to chemist William Cruikshank in 1800, it was 90 years later that the electrolytic method was used successfully on a commercial scale.
[7] In 1833, Faraday formulated the laws that governed the electrolysis of aqueous solutions, and patents were issued to Cook and Watt in 1851 and to Stanley in 1853 for the electrolytic production of chlorine from brine.
Additionally, the chlorine and sodium hydroxide produced via the mercury-cell chloralkali process are themselves contaminated with trace amounts of mercury.
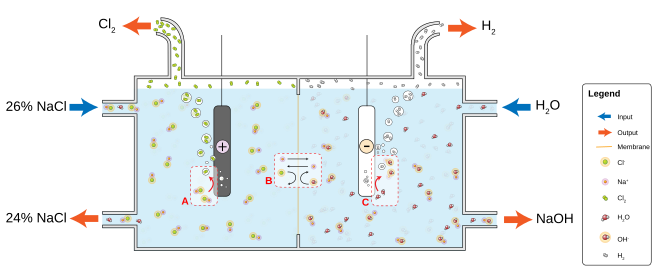
The most common chloralkali process involves the electrolysis of aqueous sodium chloride (a brine) in a membrane cell.
Similarly to the membrane cell, chloride ions are oxidized at the anode to produce chlorine, and at the cathode, water is split into caustic soda and hydrogen.
The cathode (where hydroxide forms) can be made from unalloyed titanium, graphite, or a more easily oxidized metal such as stainless steel or nickel.
The interests of chloralkali product manufacturers are represented at regional, national and international levels by associations such as Euro Chlor and The World Chlorine Council.