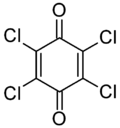
Chloranil
Like the parent benzoquinone, chloranil is a planar molecule[2] that functions as a mild oxidant.
Chloranil is produced by chlorination of phenol to give hexachlorocyclohexa-2,5-dien-1-one ("hexachlorophenol").
Hydrolysis of the dichloromethylene group in this dienone gives chloranil:[3] Chloroanil serves as a hydrogen acceptor.
It is used for the aromatization reactions, such as the conversion of cyclohexadienes to the benzene derivatives.
It is a precursor to many dyes, such as pigment violet 23 and diaziquone (AZQ), a cancer chemotherapeutic agent.