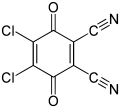
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
[5] DDQ decomposes in water, but is stable in aqueous mineral acid.
[7] The substance did not receive interest until its potential as a dehydrogenation agent was discovered.
The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene: The resulting hydroquinone is poorly soluble in typical reaction solvents (dioxane, benzene, alkanes), which facilitates workup.
Solutions of DDQ in benzene are red, due to the formation of a charge-transfer complex.
[9] DDQ reacts with water to release highly toxic hydrogen cyanide (HCN).