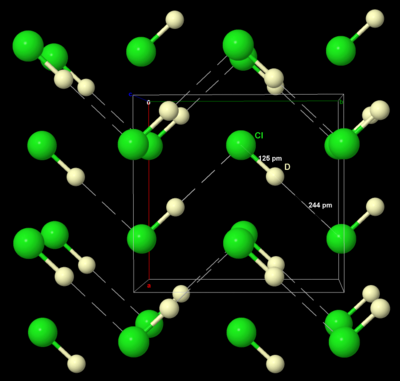
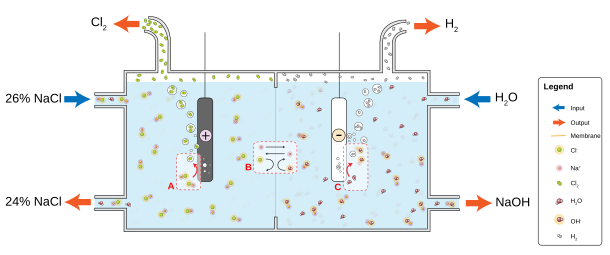
Chlorine
In 1809, chemists suggested that the gas might be a pure element, and this was confirmed by Sir Humphry Davy in 1810, who named it after the Ancient Greek χλωρός (khlōrós, "pale green") because of its colour.
The high oxidising potential of elemental chlorine led to the development of commercial bleaches and disinfectants, and a reagent for many processes in the chemical industry.
Small quantities of elemental chlorine are generated by oxidation of chloride ions in neutrophils as part of an immune system response against bacteria.
[19][20] In 1809, Joseph Louis Gay-Lussac and Louis-Jacques Thénard tried to decompose dephlogisticated muriatic acid air by reacting it with charcoal to release the free element muriaticum (and carbon dioxide).
[29] These compounds produced low levels of elemental chlorine and could be more efficiently transported than sodium hypochlorite, which remained as dilute solutions because when purified to eliminate water, it became a dangerously powerful and unstable oxidizer.
[33] Elemental chlorine solutions dissolved in chemically basic water (sodium and calcium hypochlorite) were first used as anti-putrefaction agents and disinfectants in the 1820s, in France, long before the establishment of the germ theory of disease.
[34] Elemental chlorine has since served a continuous function in topical antisepsis (wound irrigation solutions and the like) and public sanitation, particularly in swimming and drinking water.
Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising agent, reacting with many elements in order to complete its outer shell.
[42] 36Cl occurs in trace quantities in nature as a cosmogenic nuclide in a ratio of about (7–10) × 10−13 to 1 with stable chlorine isotopes: it is produced in the atmosphere by spallation of 36Ar by interactions with cosmic ray protons.
However, this trend is not shown in the bond energies because fluorine is singular due to its small size, low polarisability, and inability to show hypervalence.
[45] The simplest chlorine compound is hydrogen chloride, HCl, a major chemical in industry as well as in the laboratory, both as a gas and dissolved in water as hydrochloric acid.
The exceptions are decidedly in the minority and stem in each case from one of three causes: extreme inertness and reluctance to participate in chemical reactions (the noble gases, with the exception of xenon in the highly unstable XeCl2 and XeCl4); extreme nuclear instability hampering chemical investigation before decay and transmutation (many of the heaviest elements beyond bismuth); and having an electronegativity higher than chlorine's (oxygen and fluorine) so that the resultant binary compounds are formally not chlorides but rather oxides or fluorides of chlorine.
It will also act as a chlorofluorinating agent, adding chlorine and fluorine across a multiple bond or by oxidation: for example, it will attack carbon monoxide to form carbonyl chlorofluoride, COFCl.
It will also react exothermically with compounds containing –OH and –NH groups, such as water:[51] Chlorine trifluoride (ClF3) is a volatile colourless molecular liquid which melts at −76.3 °C and boils at 11.8 °C.
One of the most reactive chemical compounds known, the list of elements it sets on fire is diverse, containing hydrogen, potassium, phosphorus, arsenic, antimony, sulfur, selenium, tellurium, bromine, iodine, and powdered molybdenum, tungsten, rhodium, iridium, and iron.
Nickel, copper, and steel containers are usually used due to their great resistance to attack by chlorine trifluoride, stemming from the formation of an unreactive layer of metal fluoride.
Its reaction with hydrazine to form hydrogen fluoride, nitrogen, and chlorine gases was used in experimental rocket engine, but has problems largely stemming from its extreme hypergolicity resulting in ignition without any measurable delay.
[58] Dichlorine hexoxide is a dark-red liquid that freezes to form a solid which turns yellow at −180 °C: it is usually made by reaction of chlorine dioxide with oxygen.
It hydrolyses in water to give a mixture of chloric and perchloric acids: the analogous reaction with anhydrous hydrogen fluoride does not proceed to completion.
Anhydrous perchloric acid is a colourless mobile liquid that is sensitive to shock that explodes on contact with most organic compounds, sets hydrogen iodide and thionyl chloride on fire and even oxidises silver and gold.
[67] In addition, a variety of simple chlorinated hydrocarbons including dichloromethane, chloroform, and carbon tetrachloride have been isolated from marine algae.
For example, DDT, which was widely used to control insects in the mid 20th century, also accumulates in food chains, and causes reproductive problems (e.g., eggshell thinning) in certain bird species.
[72] Small batches of chlorine gas are prepared in the laboratory by combining hydrochloric acid and manganese dioxide, but the need rarely arises due to its ready availability.
In or about 1820, the Société d'encouragement pour l'industrie nationale offered a prize for the discovery of a method, chemical or mechanical, for separating the peritoneal membrane of animal intestines without putrefaction.
Labarraque's discovery helped to remove the terrible stench of decay from hospitals and dissecting rooms, and by doing so, effectively deodorised the Latin Quarter of Paris.
A modified version of this solution continues to be employed in wound irrigation in modern times, where it remains effective against bacteria that are resistant to multiple antibiotics (see Century Pharmaceuticals).
Most of the deaths were caused by the force of the explosions rather than the effects of chlorine since the toxic gas is readily dispersed and diluted in the atmosphere by the blast.
[113] Laboratory analysis of clothing and soil samples confirmed the use of chlorine gas against Kurdish Peshmerga Forces in a vehicle-borne improvised explosive device attack on 23 January 2015 at the Highway 47 Kiske Junction near Mosul.
[131][132] In the United States, the Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit for elemental chlorine at 1 ppm, or 3 mg/m3.
Several catastrophic collapses of swimming pool ceilings have occurred from chlorine-induced stress corrosion cracking of stainless steel suspension rods.