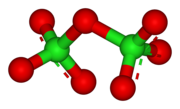
Dichlorine heptoxide
Cl2O7 is an endergonic molecule, meaning it is intrinsically unstable, decomposing to its constituent elements with release of energy:[3] Dichlorine heptoxide is a covalent compound consisting of two ClO3 portions linked by an oxygen atom.
Dichlorine heptoxide reacts with primary and secondary amines in carbon tetrachloride solution to yield perchloric amides:[4] It also reacts with alkenes to give alkyl perchlorates.
[5] Dichlorine heptoxide reacts with alcohols to form alkyl perchlorates.
[7] Nevertheless, it is less strongly oxidising than the other chlorine oxides, and does not attack sulfur, phosphorus, or paper when cold.
[1] It has the same effects on the human body as elemental chlorine, and requires the same precautions.