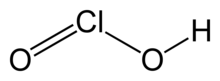
Chlorous acid
Chlorous acid is an inorganic compound with the formula HClO2.
HClO2 can be prepared through reaction of barium or lead chlorite and dilute sulfuric acid: Chlorous acid is a powerful oxidizing agent, although its tendency to undergo disproportionation counteracts its oxidizing potential.
[citation needed] Chlorine is the only halogen to form an isolable acid of formula HXO2.
[1] Fluorine is resistant to oxidation, having a -1 oxidation state even in hypofluorous acid, and is thus unable to form any higher oxoacids; despite the name, fluorite minerals are chemically fluoride compounds.
[1] Media related to Chlorous acid at Wikimedia Commons