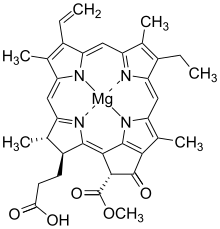
Chlorophyllide
Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway.
Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria.
[4] Details of the late stages of the biosynthetic pathway to chlorophyll differ in the plants (for example Arabidopsis thaliana, Nicotiana tabacum and Triticum aestivum) and bacteria (for example Rubrivivax gelatinosus and Synechocystis) in which it has been studied.
The metal is inserted into protoporphyrin IX by the enzyme magnesium chelatase[1] which catalyzes the reaction EC 6.6.1.1 The next step towards the chlorophyllides is the formation of a methyl (CH3) ester on one of the propionate groups, which is catalysed by the enzyme magnesium protoporphyrin IX methyltransferase[6] in the methylation reaction EC 2.1.1.11 The chlorin ring system features a five-membered carbon ring E is created when one of the propionate groups of the porphyrin is cyclised to the carbon atom linking the original pyrrole rings C and D. A series of chemical steps catalysed by the enzyme Magnesium-protoporphyrin IX monomethyl ester (oxidative) cyclase[7] gives the overall reaction EC 1.14.13.81 In barley the electrons are provided by reduced ferredoxin, which can obtain them from photosystem I or, in the dark, from Ferredoxin—NADP(+) reductase: the cyclase protein is named XanL and is encoded by the Xantha-l gene.
[8] In anaerobic organisms such as Rhodobacter sphaeroides the same overall transformation occurs but the oxygen incorporated into magnesium-protoporphyrin IX 13-monomethyl ester comes from water in the reaction EC 1.21.98.3.
The first step is the reduction (with trans stereochemistry) of the pyrrole ring B, giving the characteristic 18-electron aromatic system of many bacteriochlorophylls.