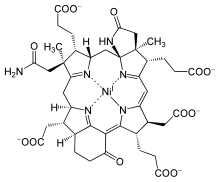
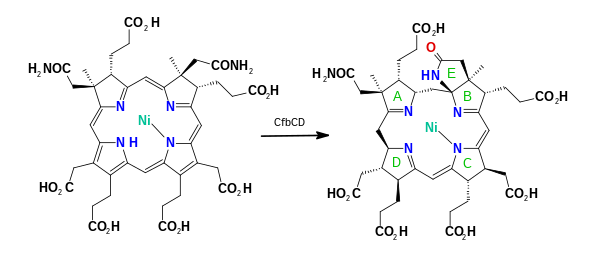
Cofactor F430
It occurs in relatively high concentrations in archaea that are involved in reverse methanogenesis: these can contain up to 7% by weight of the nickel protein.
[4] The trivial name cofactor F430 was assigned in 1978 based on the properties of a yellow sample extracted from Methanobacterium thermoautotrophicum, which had a spectroscopic maximum at 430 nm.
[5] It was identified as the MCR cofactor in 1982[6] and the complete structure was deduced by X-ray crystallography and NMR spectroscopy.
[7] Coenzyme F430 features a reduced porphyrin in a macrocyclic ring system called a corphin.
Reduction involves ATP hydrolysis and electrons are relayed through two 4Fe-4S centres.