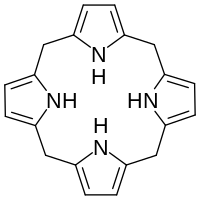
Porphyrinogen
In biochemistry, a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle of four pyrrole rings connected by four methylene bridges.
Porphyrinogens are intermediates in the biosynthesis of porphyrins, cofactors with a porphine core which are found in many enzymes and proteins including myoglobin, hemoglobin, cytochromes, and chlorophylls.
Loss of all four central hydrogen atoms in the core yields a tetravalent anion that can act as a ligand to metal cations, creating a coordination compound.
These often have side groups that do not occur in nature, and possibly at the carbons in the methylene bridges (meso positions) instead of the pyrrole rings.
[3] Alternatively, pyrrole with sidechains substituted at carbons 3 and 4 (those not adjacent to the nitrogen) can be condensed with formaldehyde H−(C=O)−H to give porphyrinogens that more closely resemble the natural ones.