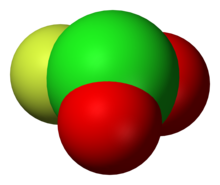
Chloryl fluoride
It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources.
[2] The compound is more conveniently prepared by reaction of sodium chlorate and chlorine trifluoride[3] and purified by vacuum fractionation, i.e. selectively condensing this species separately from other products.
This species is a gas boiling at −6 °C: In contrast to O2F2, ClO2F is a pyramidal molecule as predicted by VSEPR.
The differing structures reflects the greater tendency of chlorine to exist in positive oxidation states with oxygen and fluorine ligands.
[4] Rocket fuel chemist John Drury Clark reported in his book Ignition!