Cobalt(II) fluoride
The compound can be dissolved in warm mineral acid, and will decompose in boiling water.
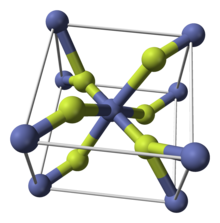
Like some other metal difluorides, CoF2 crystallizes in the rutile structure, which features octahedral Co centers and planar fluorides.
Cobalt(II) fluoride is available in most volumes in an ultra high purity composition.
To analyze this compound, Cobalt (II) fluoride can be dissolved in nitric acid.
The solution is then diluted with water until appropriate concentration for AA or ICP spectrophotometry for the cobalt.