Cockayne syndrome
Cockayne syndrome (CS), also called Neill-Dingwall syndrome, is a rare and fatal autosomal recessive neurodegenerative disorder characterized by growth failure, impaired development of the nervous system, abnormal sensitivity to sunlight (photosensitivity), eye disorders and premature aging.
[1][2][3] Failure to thrive and neurological disorders are criteria for diagnosis, while photosensitivity, hearing loss, eye abnormalities, and cavities are other very common features.
It is associated with a group of disorders called leukodystrophies, which are conditions characterized by degradation of neurological white matter.
The mutation of specific genes in Cockayne syndrome is known, but the widespread effects and its relationship with DNA repair is yet to be well understood.
In the case of this disease, due to subtle defects in transcription, children's genetic machinery for synthesizing proteins needed by the body does not operate at normal capacity.
Normally, oxidative damage repair is faster in the active genes (which make up less than five percent of the genome) than in inactive regions of the DNA.
[12] Cockayne syndrome is classified genetically as follows:[13] In contrast to cells with normal repair capability, CSA and CSB deficient cells are unable to preferentially repair cyclobutane pyrimidine dimers induced by the action of ultraviolet (UV) light on the template strand of actively transcribed genes.
[15] People with this syndrome have smaller than normal head sizes (microcephaly), are of short stature (dwarfism), their eyes appear sunken, and they have an "aged" look.
[9] The skin of those with Cockayne syndrome is also frequently affected: hyperpigmentation, varicose or spider veins (telangiectasia),[9] and serious sensitivity to sunlight are common, even in individuals without XP-CS.
Diagnosis is determined by a specific test for DNA repair, which measures the recovery of RNA after exposure to UV radiation.
For example, skeletal radiography, endocrinologic tests, and chromosomal breakage studies can help in excluding disorders included in the differential diagnosis.
[citation needed] Brain CT scanning in Cockayne syndrome patients may reveal calcifications and cortical atrophy.
[citation needed] Imaging studies reveal a widespread absence of the myelin sheaths of the neurons in the white matter of the brain and general atrophy of the cortex.
[6] Calcifications have also been found in the putamen, an area of the forebrain that regulates movements and aids in some forms of learning,[9] along with the cortex.
[7] Additionally, atrophy of the central area of the cerebellum found in patients with Cockayne syndrome could also result in the lack of muscle control, particularly involuntary, and poor posture typically seen.
[3] Also wearing high-factor sunscreen and protective clothing is recommended because Cockayne Syndrome patients are very sensitive to UV radiation.
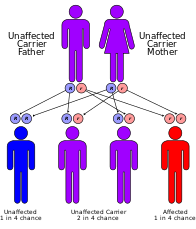
Genetic counseling for the parents is recommended, as the disorder has a 25% chance of being passed to any future children, and prenatal testing is also a possibility.
Identification of gene defects involved makes it possible to offer genetic counseling and antenatal diagnostic testing to the parents who already have one affected child.
The first project, led by the Viljem Julijan Association for Children with Rare Diseases, aims to develop gene therapy specifically for Cockayne syndrome type B.
[23] The second project, led by the Riaan Research Initiative, is dedicated to the development of gene therapy for Cockayne syndrome type A.
[15] The recent research on Jan 2018 mentions different CS features that are seen globally with similarities and differences: CS has an incidence of 1 in 250,000 live births, and a prevalence of approximately 1 per 2.5 million, which is remarkably consistent across various regions globally:[26][27] Calcification [55–95%] of the cerebral cortex (especially depths of sulci, basal ganglia, cerebellum, thalamus; also of the arteries, arterioles, and capillaries).
Amyloid plaques, neurofibrillary tangles, Hirano bodies not commonly seen, although ubiquitin reactivity of axons present Cataracts [36–86%].