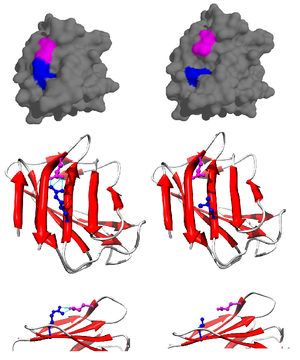
Prelamin-A/C
[8] In the setting of ZMPSTE24 deficiency, the final step of lamin processing does not occur, resulting in an accumulation of farnesyl-prelamin A.
Consequently, no mature lamin A is formed, and a farnesylated mutant prelamin A (progerin) accumulates in cells.
Lamin proteins are thought to be involved in nuclear stability, chromatin structure and gene expression.
DNA double-strand damages can be repaired by either homologous recombination (HR) or non-homologous end joining (NHEJ).
LMNA promotes genetic stability by maintaining the levels of proteins that have key roles in HR and NHEJ.