Cooperativity
Cooperativity is a phenomenon displayed by systems involving identical or near-identical elements, which act dependently of each other, relative to a hypothetical standard non-interacting system in which the individual elements are acting independently.
[1] We also see cooperativity in large chain molecules made of many identical (or nearly identical) subunits (such as DNA, proteins, and phospholipids), when such molecules undergo phase transitions such as melting, unfolding or unwinding.
However, the definition of cooperativity based on apparent increase or decrease in affinity to successive ligand binding steps is problematic, as the concept of "energy" must always be defined relative to a standard state.
A much more general and useful definition of positive cooperativity is: A process involving multiple identical incremental steps, in which intermediate states are statistically underrepresented relative to a hypothetical standard system (null hypothesis) where the steps occur independently of each other.
Likewise, a definition of negative cooperativity would be a process involving multiple identical incremental steps, in which the intermediate states are overrepresented relative to a hypothetical standard state in which individual steps occur independently.
[2] These latter definitions for positive and negative cooperativity easily encompass all processes which we call "cooperative", including conformational transitions in large molecules (such as proteins) and even psychological phenomena of large numbers of people (which can act independently of each other, or in a co-operative fashion).
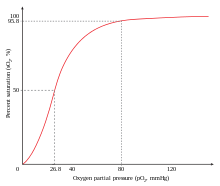
Deoxy-hemoglobin has a relatively low affinity for oxygen, but when one molecule binds to a single heme, the oxygen affinity increases, allowing the second molecule to bind more easily, and the third and fourth even more easily.
This behavior leads the affinity curve of hemoglobin to be sigmoidal, rather than hyperbolic as with the monomeric myoglobin.
Homotropic or heterotropic cooperativity could be of both positives as well as negative types depend upon whether it support or oppose further binding of the ligand molecules to the enzymes.
[3] Cooperativity is not only a phenomenon of ligand binding, but also applies anytime energetic interactions make it easier or more difficult for something to happen involving multiple units as opposed to with single units.
(That is, easier or more difficult compared with what is expected when only accounting for the addition of multiple units).
A simple and widely used model for molecular interactions is the Hill equation, which provides a way to quantify cooperative binding by describing the fraction of saturated ligand binding sites as a function of the ligand concentration.
Global sensitivity measures such as the Hill coefficient do not characterise the local behaviours of the s-shaped curves.
Consider two coupled ultrasensitive modules, disregarding effects of sequestration of molecular components between layers.
In this case, the expression for the system's dose-response curve, F, results from the mathematical composition of the functions,
Altszyler et al. (2017) [5] have shown that the cascade's global ultrasensitivity can be analytically calculated: where
, which characterized local average sensitivities over the relevant input region for each layer:
For the more general case of a cascade of N modules, the Hill coefficient can be expressed as: Several authors have reported the existence of supramultiplicative behavior in signaling cascades [8][9](i.e. the ultrasensitivity of the combination of layers is higher than the product of individual ultrasensitivities), but in many cases the ultimate origin of supramultiplicativity remained elusive.
Altszyler et al. (2017)[5] framework naturally suggested a general scenario where supramultiplicative behavior could take place.
This could occur when, for a given module, the corresponding Hill's input working range was located in an input region with local ultrasensitivities higher than the global ultrasensitivity of the respective dose-response curve.