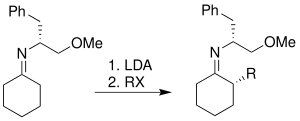
Enders SAMP/RAMP hydrazone-alkylation reaction
[3] This reaction is a useful technique for asymmetric α-alkylation of ketones and aldehydes, which are common synthetic intermediates for medicinally interesting natural products and other related organic compounds.
These natural products include (-)-C10-demethyl arteannuin B, the structural analog of antimalarial artemisinin,[4] the polypropionate metabolite (-)-denticulatin A and B isolated from Siphonaria denticulata,[5] zaragozic acid A, a potent inhibitor of sterol synthesis,[6] and epothilone A and B, which have been proven to be very effective anticancer drugs.
[7] Regioselective and stereoselective formation of carbon-carbon bonds adjacent to carbonyl group is an important procedure in organic chemistry.
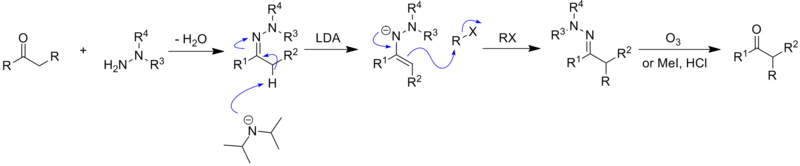
[10] In 1976, Meyers reported the first alkylation reaction of metallated azaenolates of hydrazones with an acyclic amino acid-based auxiliary.
Compared with the free carbonyl compounds and the chiral enamine species reported previously, the hydrazones exhibit higher reactivity, regioselectivity and stereoselectivity.
[18] The chelation of lithium cation with the methoxy group is one of the most important features of the transition state for Enders' hydrazone alkylation reaction.
The development and modification of Enders' hydrazone alkylation reaction mainly focus on the addition of more steric hindrance on the pyrrolidine rings of both SAMP and RAMP, while preserving the methoxy group for lithium chelation.
These new compounds consist of a new class of chiral auxiliary based on the carbamate structure and, therefore, no longer belong to the family of SAMP and RAMP.
Ozone or singlet oxygen can ozonolyze the diazene bond (and any olefinic moieties present), leaving a carbonyl, a nitrosamine, and dioxygen.
Peroxide reagents (e.g. NaBO3, (tBu4NSO4)2, or m-ClBzO2H) cleave the hydrazone with varying speeds, selectivities, and mechanisms, but the Baeyer-Villiger oxidation is a common side-reaction.
All except ozone and singlet oxygen generate nitriles from aldehydic hydrazones, either as the major or a substantial minor product.
Silica gel hydrolyzes exquisitely acid-sensitive substrates, but is too weak to affect ketonic hydrazones adjacent to a primary carbon.
Ketonic hydrazones adjacent to a secondary or tertiary carbon hydrolyze in the presence of catalytic cupric salts; that procedure also preserves substrates disturbed by oxidants or strong acids.
After 12 hours of reaction at room temperature, the crude alkylated hydrazone is allowed to react with ozone in a Schlenk tube to cleave the C=N bond.
The subsequent ozonolysis and Wittig reaction led to the side chain fragment of zaragozic acid A, which is a potent medicine for coronary heart disease.
To avoid loss of the enantiomeric purity of the product, the authors used cupric acetate to regenerate the carbonyl group, obtaining only moderate yield for the cleavage of C=N bond but good enantioselectivity (ee = 89%).
Several of their structural derivatives show very promising inhibition against breast cancer with only mild side effect and some of them are now under trials.