Electrophile
Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL.
Common reactions include use of bromine water to titrate against a sample to deduce the number of double bonds present.
For example, ethene + bromine → 1,2-dibromoethane: This takes the form of 3 main steps shown below;[3] This process is called AdE2 mechanism ("addition, electrophilic, second-order").
An example is shown below: In this manner, the stereoselectivity of the product, that is, from which side Cl− will attack relies on the types of alkenes applied and conditions of the reaction.
Hydrogen bromide (HBr) also takes this pathway, but sometimes a radical process competes and a mixture of isomers may form.
The extent to which each pathway contributes depends on the several factors like the nature of the solvent (e.g., polarity), nucleophilicity of the halide ion, stability of the carbocation, and steric effects.
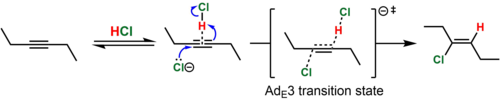
[7] In the case of dialkyl-substituted alkynes (e.g., 3-hexyne), the intermediate vinyl cation that would result from this process is highly unstable.
Because the simultaneous collision of three chemical species in a reactive orientation is improbable, the termolecular transition state is believed to be reached when the nucleophile attacks a reversibly-formed weak association of the alkyne and HCl.
The proximity of the anion to the side of the vinyl cation where the proton was added is used to rationalize the observed predominance of syn addition.
This is an important reaction in industry, as it produces ethanol, whose purposes include fuels and starting material for other chemicals.
The Shi catalyst, a ketone, is oxidized by stoichiometric oxone to the active dioxirane form before proceeding in the catalytic cycle.
Oxaziridines such as chiral N-sulfonyloxaziridines effect enantioselective ketone alpha oxidation en route to the AB-ring segments of various natural products, including γ-rhodomycionone and α-citromycinone.
Solid-supported reagents offers advantages over solution phase chemistry due to the ease of workup and purification.
Correlations have been found between electrophilicity of various chemical compounds and reaction rates in biochemical systems and such phenomena as allergic contact dermititis.
[citation needed] Superelectrophiles are defined as cationic electrophilic reagents with greatly enhanced reactivities in the presence of superacids.