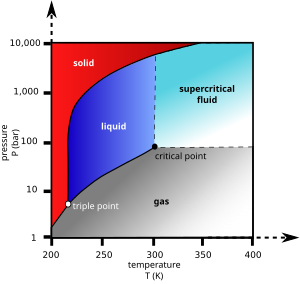
Supercritical drying
Delicate structures such as cell walls, the dendrites in silica gel, and the tiny machinery of microelectromechanical devices, tend to be broken apart by this surface tension as the liquid–gas–solid junction moves by.
Supercritical drying, on the other hand, goes around the line to the right, on the high-temperature, high-pressure side (red arrow).
In most such processes, acetone is first used to wash away all water, exploiting the complete miscibility of these two fluids.
The acetone is then washed away with high pressure liquid carbon dioxide, the industry standard now that freon is unavailable.
The liquid carbon dioxide is then heated until its temperature goes beyond the critical point, at which time the pressure can be gradually released, allowing the gas to escape and leaving a dried product.