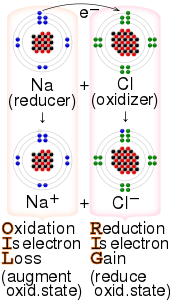
Oxidizing agent
In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate.
In some cases, these oxides can also serve as electron acceptors, as illustrated by the conversion of MnO−4 to MnO2−4,ie permanganate to manganate.
The dangerous goods definition of an oxidizing agent is a substance that can cause or contribute to the combustion of other material.
An example is potassium dichromate, which does not pass the dangerous goods test of an oxidizing agent.
5.1(a)2 of the DOT code applies to liquid oxidizers "if, when tested in accordance with the UN Manual of Tests and Criteria, it spontaneously ignites or its mean time for a pressure rise from 690 kPa to 2070 kPa gauge is less than the time of a 1:1 nitric acid (65 percent)/cellulose mixture.