Cucurbituril
The oxygen atoms are located along the edges of the band and are tilted inwards, forming a partly enclosed cavity (cavitand).
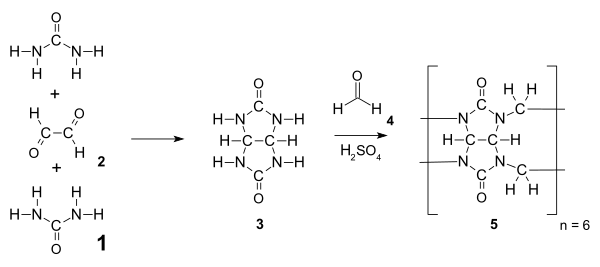
Cucurbiturils are amidals (less precisely aminals) and synthesized from urea 1 and a dialdehyde (e.g., glyoxal 2) via a nucleophilic addition to give the intermediate glycoluril 3.
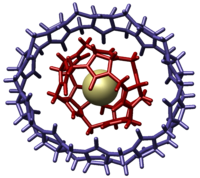
It was first discovered by Day and coworkers in 2002 as an inclusion complex containing CB[5] by fractional crystallization of the cucurbituril reaction mixture.
[7] The CB[10]·CB[5] was unambiguously identified by single crystal X-ray structural analysis that revealed the complex resembled a molecular gyroscope.
Cucurbiturils have been used by chemists for various applications, including drug delivery, asymmetric synthesis, molecular switching, and dye tuning.
Cucurbiturils are efficient host molecules in molecular recognition and have a particularly high affinity for positively charged or cationic compounds.
High association constants with positively charged molecules are attributed to the carbonyl groups that line each end of the cavity and can interact with cations in a similar fashion to crown ethers.
For example, the affinity equilibrium constant of cucurbit[7]uril with the positively charged 1-aminoadamantane hydrochloride is experimentally determined at 4.23*1012.
Given their high affinities to form inclusion complexes cucurbiturils have been employed as the macrocycles component of a rotaxane.
[12] In another rotaxane system with a CB[7] wheel, the axle is a 4,4'-bipyridinium or viologen subunit with two carboxylic acid terminated aliphatic N-substituents at both ends.
[15] The potential of this application has been explored with cucurbit[7]uril that forms an inclusion compound with the important cancer fighting drug oxaliplatin.
CB[7] was employed despite the fact that it is more difficult to isolate since it has much greater solubility in water and its larger cavity size can accommodate the drug molecule.
The resulting complex was found to have increased stability and greater selectivity that may lead to fewer side effects.
[17] The close proximity and optimal orientation of the guest molecules within the cavity enhances the rate of the photochemical cyclization to give cyclobutane dimer with a 19:1 stereoselectivity for the syn configuration when bound to CB[8].