Thermodynamic versus kinetic reaction control
Because pairs of enantiomers have, for all intents and purposes, the same Gibbs free energy, thermodynamic control will produce a racemic mixture by necessity.
Thus, any catalytic reaction that provides product with nonzero enantiomeric excess is under at least partial kinetic control.
At 81 °C and after long reaction times, the chemical equilibrium can assert itself and the thermodynamically more stable exo isomer 1 is formed.
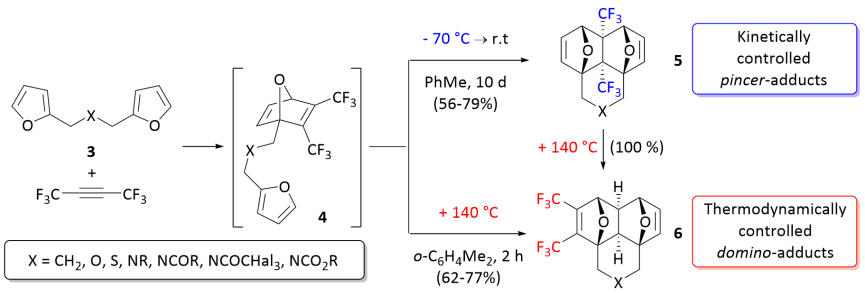
[5][6] At low temperature, the reactions occur chemoselectively leading exclusively to adducts of pincer-[4+2] cycloaddition (5).
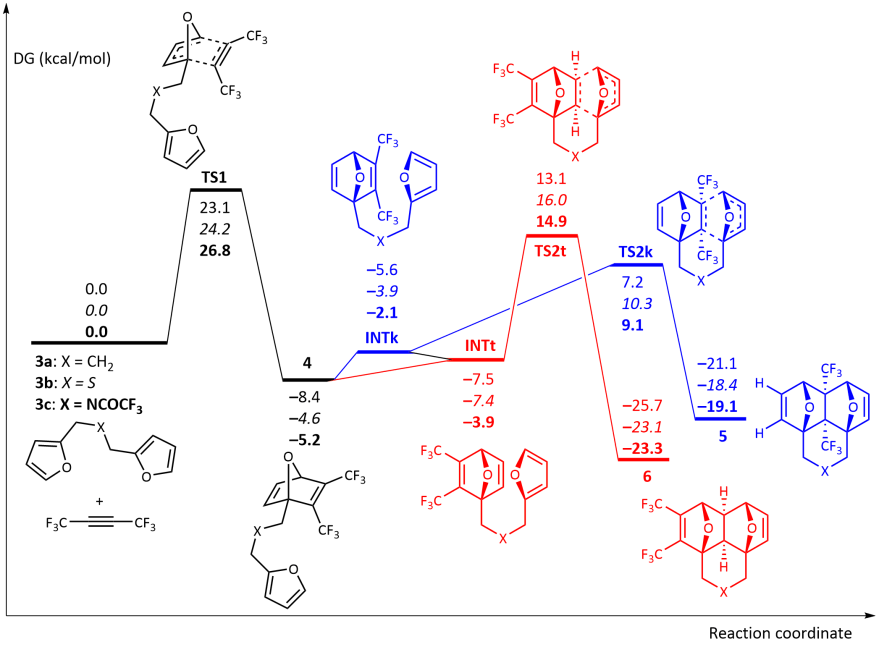
The reaction starting with [4+2] cycloaddition of CF3C≡CCF3 at one of the furan moieties occurs in a concerted fashion via TS1 and represents the rate limiting step of the whole process with the activation barrier ΔG‡ ≈ 23.1–26.8 kcal/mol.
Indeed, the calculated activation barriers for the 5 → 6 isomerization via the retro-Diels–Alder reaction of 5 followed by the intramolecular [4+2]-cycloaddition in the chain intermediate 4 to give 6 are 34.0–34.4 kcal/mol.
[18] They were re-investigating a reaction between maleic anhydride and a fulvene first reported in 1929 by Otto Diels and Kurt Alder.
This was interpreted as a case in the field of anionotropy of the phenomenon, familiar in prototropy, of the distinction between kinetic and thermodynamic control in ion-recombination.