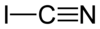Cyanogen iodide
It occurs as white crystals that react slowly with water to form hydrogen cyanide.
[citation needed] Cyanogen iodide is toxic if inhaled or ingested and may be fatal if swallowed or absorbed through the skin.
ICN slowly reacts with water or carbon dioxide to produce hydrogen cyanide.
11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
Results confirm that cyanides are much weaker salts in pyridine than are iodides, although cyanogen iodide solutions are able to be dissolved in pyridine giving solutions with electrical conductivity that increases over time and results in maximum values.