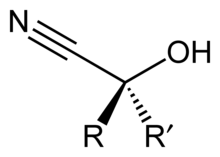
Cyanohydrin
Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst:[1] In this reaction, the nucleophilic CN− ion attacks the electrophilic carbonyl carbon in the ketone, followed by protonation by HCN, thereby regenerating the cyanide anion.
[5] On a laboratory scale the use of HCN (toxic) is largely not encouraged, for this reason other less dangerous cyanation reagents are sought out.
[6] Similar procedures relying on ester, phosphate and carbonate formation have been reported.
[7][8][9] Mandelonitrile, with the formula C6H5CH(OH)CN, occurs in small amounts in the pits of some fruits.
[1] Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN.