Gattermann reaction
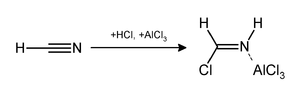
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as aluminium chloride (AlCl3).
[1] It is named for the German chemist Ludwig Gattermann[2] and is similar to the Friedel–Crafts reaction.
[4] Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN.
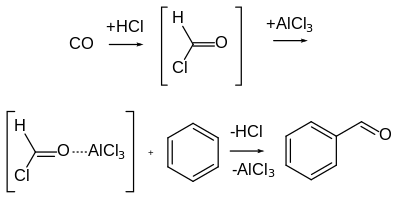
[6] The Gattermann–Koch reaction, named after the German chemists Ludwig Gattermann and Julius Arnold Koch,[7] is a variant of the Gattermann reaction in which carbon monoxide (CO) is used instead of hydrogen cyanide.
The transition metal co-catalyst may server as a "carrier" by first reacting with CO to form a carbonyl complex, which is then transformed into the active electrophile.