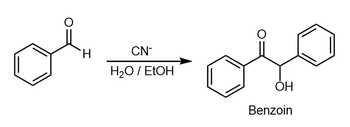
Benzoin condensation
The reaction generally occurs between aromatic aldehydes or glyoxals (OCH=CHO),[1][2] and results in formation of an acyloin (−C(O)CH(OH)−).
[3] The benzoin condensation was first reported in 1832 by Justus von Liebig and Friedrich Wöhler during their research on bitter almond oil.
In biochemistry, the coenzyme thiamine is responsible for biosynthesis of acyloin-like compounds utilizing the benzoin addition.
The asymmetric version of this reaction has been performed by utilizing chiral thiazolium and triazolium salts.
If a benzoin or acyloin can be synthesized by another method, then they can be converted into the component ketones using cyanide or thiazolium catalysts.