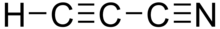Cyanopolyyne
Structurally, they are polyynes with a cyano group (−C≡N) covalently bonded to one of the terminal acetylene units (H−C≡C).
Cyanoacetylene is more common on Earth and it is believed to be the initial reagent for most of the photocatalyzed formation of the interstellar cyanopolyynes.
The calculations by Winstanley show that photoionization and dissociation reactions play a profound role in the abundances of cyanopolyynes after about 1 million years.
However, the fractional abundances of cyanopolyyne are less affected by changes in radiation field intensity past time 1 million years because the prevailing neutral-neutral reactions surpass the effects of photoreactions.
As with many other molecules the cyanopolyynes are detected with a spectrometer which records the quantum energy levels of the electrons within the atoms.
The detection process usually happens within the outer ranges of the electromagnetic spectrum, usually in infrared or radio waves.
Such places include the atmosphere on Titan and the gas clouds that are within nebulae and the confines of dying stars.
[9] Species as large as HC9N were detected in Taurus Molecular Cloud 1, where they are believed to be formed by reaction of atomic nitrogen with hydrocarbons.