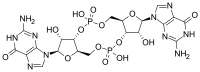
Cyclic di-GMP
These proteins typically have a characteristic GGDEF motif, which refers to a conserved sequence of five amino acids.
Processes that are known to be regulated by cyclic di-GMP, at least in some organisms, include biofilm formation (such as EPS matrices found by Steiner et al 2013),[4] motility (especially the motile-to-sessile transition, see the review by Jenal et al 2017)[4] and virulence factor production.
Enzymes that degrade or synthesize cyclic di-GMP are believed to be localized to specific regions of the cell, where they influence receivers in a restricted space.
[5][6] This leads to the strong inference that conformational changes in PilZ domains allow the activity of targeted effector proteins (such as cellulose synthase) to be regulated by cyclic di-GMP.
Genes wspE and wspF from the wsp operon exhibited mutations that upregulated c-di-GMP.