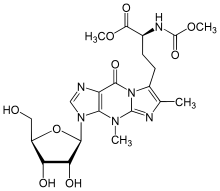
Wybutosine
In biochemistry, wybutosine (yW) is a heavily modified nucleoside of phenylalanine transfer RNA that stabilizes interactions between the codons and anti-codons during protein synthesis.
Proceeding through a multi-enzymatic process, the first step of the synthesis involves the enzyme N1-methyltransferase TRM5 which methylates the G37 site of phenylalanine tRNA and converts it to m1G37.
Then m1G37 acts as a substrate for the enzyme TYW1 and, using pyruvate as a C-3 source, forms the tricyclic core of wybutosine with flavin mononucleotide (FMN) as a cofactor.
[7] Through its large aromatic groups, stacking interactions with adjacent bases A36 and A38 are enhanced, which help to restrict the flexibility of the anticodon.
The fact that wybutosine and its various derivatives are only found at position 37 may be indicative of the nature of the phenylalanine codons, UUU and UUC, and their predilection for ribosome slippage.