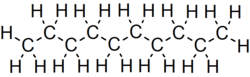Decane
Although 75 structural isomers are possible for decane, the term usually refers to the normal-decane ("n-decane"), with the formula CH3(CH2)8CH3.
Decane is present in small quantities (less than 1%) in gasoline (petrol) and kerosene.
[6][7] Like other alkanes, it is a nonpolar solvent, and does not dissolve in water, and is readily combustible.
Although it is a component of fuels, it is of little importance as a chemical feedstock, unlike a handful of other alkanes.
In the presence of sufficient oxygen, it burns to form water and carbon dioxide.