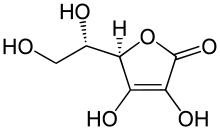
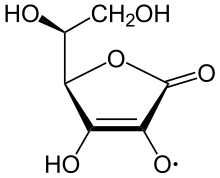
Chemistry of ascorbic acid
The l isomer is the one most often encountered: it occurs naturally in many foods, and is one form ("vitamer") of vitamin C, an essential nutrient for humans and many animals.
In 1907, Axel Holst and Theodor Frølich discovered that the antiscorbutic factor was a water-soluble chemical substance, distinct from the one that prevented beriberi.
Between 1928 and 1932, Albert Szent-Györgyi isolated a candidate for this substance, which he called "hexuronic acid", first from plants and later from animal adrenal glands.
In 1933, sugar chemist Walter Norman Haworth, working with samples of "hexuronic acid" that Szent-Györgyi had isolated from paprika and sent him in the previous year, deduced the correct structure and optical-isomeric nature of the compound, and in 1934 reported its first synthesis.
It is a mild reducing agent and antioxidant, typically reacting with oxidants of the reactive oxygen species, such as the hydroxyl radical.
Reactive oxygen species are damaging to animals and plants at the molecular level due to their possible interaction with nucleic acids, proteins, and lipids.
[9] It is a cofactor in tyrosine oxidation, though because a crude extract of animal liver is used, it is unclear which reaction catalyzed by which enzyme is being helped here.
[14][15][16] Research conducted in the 1960s suggested ascorbic acid could substantially contribute to urinary oxalate content (possibly over 40%), but these estimates have been questioned due to methodological limitations.
[14][15][17] Subsequent large cohort studies have yielded conflicting results regarding the link between vitamin C intake and kidney stone formation.
The overall clinical significance of ascorbic acid consumption to kidney stone risk, however, remains inconclusive, although several studies have suggested a potential association, especially with high-dose supplementation in men.
[14][15][18][19] The main use of l-ascorbic acid and its salts is as food additives, mostly to combat oxidation and prevent discoloration of the product during storage.
[25][26] Its deficiency over a prolonged period of time could cause scurvy, which is characterized by fatigue, widespread weakness in connective tissues and capillary fragility.
[27] It affects multiple organ systems due to its role in the biochemical reactions of connective tissue synthesis.
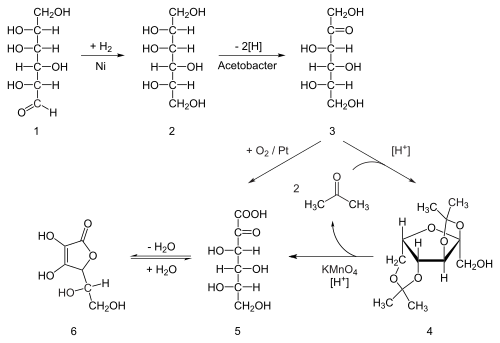
Acid-catalyzed hydrolysis of this product performs the dual function of removing the two acetal groups and ring-closing lactonization.
A second genetically modified microbe species, such as mutant Erwinia, among others, oxidises sorbose into 2-ketogluconic acid (2-KGA), which can then undergo ring-closing lactonization via dehydration.
As an alternative, ascorbic acid can be treated with iodine in excess, followed by back titration with sodium thiosulfate using starch as an indicator.