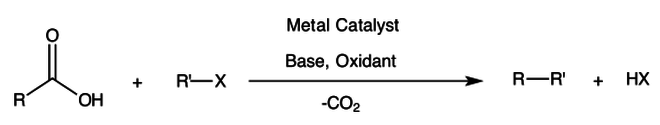
Decarboxylative cross-coupling
A significant advantage of this reaction is that it uses relatively inexpensive carboxylic acids (or their salts) and is far less air and moisture sensitive in comparison to typical cross-coupling organometallic reagents.
Furthermore, the carboxylic acid moiety is a common feature of natural products and can also be prepared by relatively benign air oxidations.
Thermal decarboxylation of copper benzoates, in the presence of an aryl halide, was found to produce (both symmetric and unsymmetric) biaryls through aryl-Cu intermediates.
[2] This monometallic copper system required drastic conditions for complete cross-coupling, and had various intrinsic limitations, both of which prevented development of a catalytic, preparatory version of this reaction.
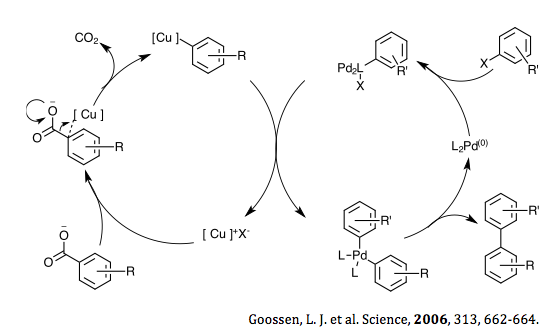
[4] A Pd–Cu bimetallic system was not discovered until 2006 when Goossen et al. reported a decarboxylative cross-coupling of aryl halides with ortho-substituted aromatic carboxylic acids.
[14] Through subsequent studies it was found that the use of aryl triflates allowed substrate scope for cross-coupling to be extended to some aromatic carboxylates lacking any ortho-substitution (less reactive).
[3] As well, the variability of this combined catalytic system allows for promotion of a large spectrum of reactions, including aryl ketone formation, c-heteroatom cross-coupling, and many others.
[3][18] The product scope of this reaction is extremely broad with the use of different substrates; however development of different functionalities has required accompanied studies to determine the proper catalyst system.
[1] Per IUPAC, the term biaryl refers to an assembly of two aromatic rings joined by a single bond,[19] starting with the simplest, biphenyl.
Biaryls constitute an important structural motif of physical organic, synthetic, and catalytic interest—for instance, underlying the area of atropisomers in enantioselective synthesis—and they appear in many pharmaceutical, agrochemical, and materials (e.g. LCD) applications.
[21] [22] [23] [24] Further work by Goossen et al. described the synthesis of ketones from α-oxocarboxylic acids with aryl or heteroaryl bromides through an acyl anion intermediate.
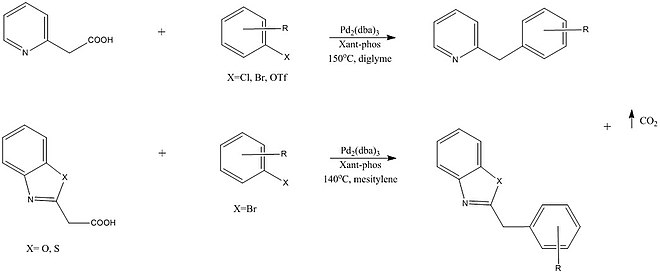
One such reaction by Shang et al. described a palladium catalyzed cross coupling that enables the formation of functionalized pyridines, pyrazines, quinolines, benzothiazoles, and benzoxazoles.
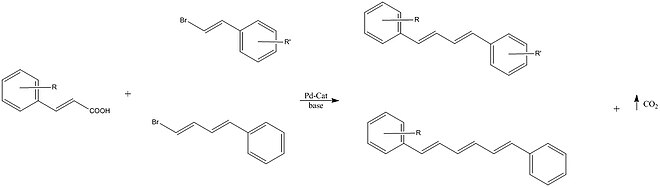
[26] Miura et al. reported the cross coupling of vinyl bromides with an alkenyl carboxylic acid using a palladium catalyst.
[30] Liu et al. reported the C-S coupling of aryl carboxylic acids with disulphides or thiols using a Pd/Cu catalyst system.
This proton is abstracted by silver carbonate, which acts as both a base and an oxidant to regenerate the starting palladium complex completing the catalytic cycle.
Forgione, P., Bilodeau, F. et al. reported that heteroatoms containing a carboxylic acid also are tolerated by palladium monometallic systems and undergo decarboxylative cross coupling with aryl halides.