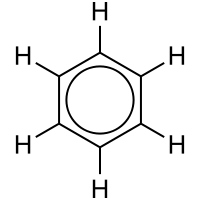
Delocalized electron
[1] The term delocalization is general and can have slightly different meanings in different fields: In the simple aromatic ring of benzene, the delocalization of six π electrons over the C6 ring is often graphically indicated by a circle.
In valence bond theory, delocalization in benzene is represented by resonance structures.
Metallic structure consists of aligned positive ions (cations) in a "sea" of delocalized electrons.
This means that the electrons are free to move throughout the structure, and gives rise to properties such as conductivity.
In the methane molecule, ab initio calculations show bonding character in four molecular orbitals, sharing the electrons uniformly among all five atoms.