Determination of equilibrium constants
For this assumption to be valid, equilibrium constants must be determined in a medium of relatively high ionic strength.
[2] When the electrode is calibrated with solutions of known concentration, by means of a strong acid–strong base titration, for example, a modified Nernst equation is assumed.
In principle absorbance may be measured at one wavelength only, but in present-day practice it is common to record complete spectra.
An upper limit on log10 β of 4 is usually quoted, corresponding to the precision of the measurements, but it also depends on how intense the effect is.
The magnitude of the constant φ may be higher than the value of the molar extinction coefficient, ε, for a species.
Example: the pKa of the hydroxyl group in citric acid has been determined from 13C chemical shift data to be 14.4.
A ligand C is chosen which forms a weaker complex with A The stability constant, KAC, is small enough to be determined by a direct method.
A general computational procedure has four main components: The value of the equilibrium constant for the formation of a 1:1 complex, such as a host-guest species, may be calculated with a dedicated spreadsheet application, Bindfit:[4] In this case step 2 can be performed with a non-iterative procedure and the pre-programmed routine Solver can be used for step 3.
In general, solving these nonlinear equations presents a formidable challenge because of the huge range over which the free concentrations may vary.
Note that the free reactant concentrations can be regarded as implicit parameters in the equilibrium constant refinement process.
In that context the values of the free concentrations are constrained by forcing the conditions of mass-balance to apply at all stages of the process.
The objective of the refinement process is to find equilibrium constant values that give the best fit to the experimental data.
Unit weights, Wii = 1, are often used but, in that case, the expectation value of U is the root mean square of the experimental errors.
At the minimum the derivatives ∂U/∂pi, which are simply related to the elements of the Jacobian matrix, J where pk is the kth parameter of the refinement, are equal to zero.
The parameter increments δp are calculated by solving the normal equations, derived from the conditions that ∂U/∂p = 0 at the minimum.
If, however, the updated parameters do not result in a decrease of the objective function, that is, if divergence occurs, the increment calculation must be modified.
With the more powerful Levenberg–Marquardt algorithm, on the other hand, the shift vector is rotated towards the direction of steepest descent, by modifying the normal equations, where λ is the Marquardt parameter and I is an identity matrix.
In this case σ2 is approximated by where U is the minimum value of the objective function and nd and np are the number of data and parameters, respectively.
The stepwise association constant for formation of LH3 is given by Substitute the expressions for the concentrations of LH3 and LH−2 into this equation whence and since pKa = −log10 1/K its value is given by Note the reverse numbering for pK and log β.
By error propagation and Once a refinement has been completed the results should be checked to verify that the chosen model is acceptable.
generally speaking, a model is acceptable when the data are fitted within experimental error, but there is no single criterion to use to make the judgement.
[8] It is therefore very useful to estimate experimental errors and derive some reasonable weights from them as this is an absolute indicator of the goodness of fit.
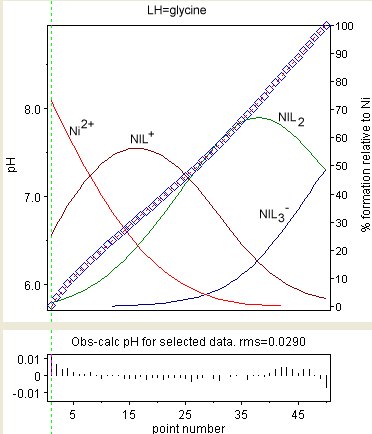
The observed values are shown a blue diamonds and the species concentrations, as a percentage of the total nickel, are superimposed.
Hydrolysis constants of metal ions are usually fixed at values which were obtained using ligand-free solutions.
Use of such constraints reduces the number of parameters to be determined, but may result in the calculated errors on refined stability constant values being under-estimated.
Such interactions are studied in an aqueous media utilizing synthetic organometallic hosts and organic guest molecules.
For example, a poly-cationic receptor containing copper (the host) is coordinated with molecules such as tetracarboxylates, tricarballate, aspartate, and acetate (the guests).
This study illustrates that entropy rather than enthalpy determines the binding energy of the system leading to negative cooperativity.
The large change in entropy originates from the displacement of solvent molecules surrounding the ligand and the receptor.
[12] In a similar study, utilizing guanidinium and Cu(II) and polycarboxylate guests, it is demonstrated that positive cooperatively is largely determined by enthalpy.