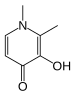
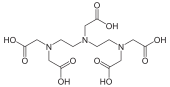
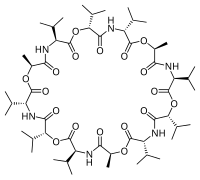
Stability constants of complexes
The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution.
[1] The reasons why this occurred at such a late date, nearly 50 years after Alfred Werner had proposed the correct structures for coordination complexes, have been summarised by Beck and Nagypál.
[2] The key to Bjerrum's method was the use of the then recently developed glass electrode and pH meter to determine the concentration of hydrogen ions in solution.
Subsequently, computer programs capable of handling complex equilibria in general, such as SCOGS[6] and MINIQUAD[7] were developed so that today the determination of stability constants has almost become a "routine" operation.
For more details see: acid–base reaction, acid catalysis, Extraction (chemistry) The thermodynamics of metal ion complex formation provides much significant information.
The standard enthalpy change can be determined by calorimetry or by using the Van 't Hoff equation, though the calorimetric method is preferable.
The fact that stepwise formation constants of complexes of the type MLn decrease in magnitude as n increases may be partly explained in terms of the entropy factor.
Another example involves iron(III) which forms weak complexes with halide and other anions, but not with perchlorate ions.
Consider the two equilibria, in aqueous solution, between the copper(II) ion, Cu2+ and ethylenediamine (en) on the one hand and methylamine, MeNH2 on the other.
Removal of a proton from an aliphatic –OH group is difficult to achieve in aqueous solution because the energy required for this process is rather large.
[24] This effect contributes the ability of hemoglobin to bind oxygen reversibly under biological conditions.
The reason for this is that, in aqueous solution, the ion written as Ag+ actually exists as the four-coordinate tetrahedral aqua species [Ag(H2O)4]+.
The first step is then a substitution reaction involving the displacement of a bound water molecule by ammonia forming the tetrahedral complex [Ag(NH3)(H2O)3]+.
Ahrland, Chatt and Davies proposed that metal ions could be described as class A if they formed stronger complexes with ligands whose donor atoms are nitrogen, oxygen or fluorine than with ligands whose donor atoms are phosphorus, sulfur or chlorine and class B if the reverse is true.
Chelation therapy is used in the treatment of various metal-related illnesses, such as iron overload in β-thalassemia sufferers who have been given blood transfusions.
The ideal ligand binds to the target metal ion and not to others, but this degree of selectivity is very hard to achieve.
The synthetic drug deferiprone achieves selectivity by having two oxygen donor atoms so that it binds to Fe3+ in preference to any of the other divalent ions that are present in the human body, such as Mg2+, Ca2+ and Zn2+.
The large stability constant of the octadentate ligand ensures that the concentration of free Gd3+ is almost negligible, certainly well below toxicity threshold.
The ninth site is occupied by a water molecule which exchanges rapidly with the fluid surrounding it and it is this mechanism that makes the paramagnetic complex into a contrast reagent.
For example, it is often present in washing powder to act as a water softener by sequestering calcium and magnesium ions.
For example, potassium selective electrodes are available that make use of the naturally occurring macrocyclic antibiotic valinomycin.
An ion-exchange resin such as chelex 100, which contains chelating ligands bound to a polymer, can be used in water softeners and in chromatographic separation techniques.
In phase-transfer catalysis, a substance which is insoluble in an organic solvent can be made soluble by addition of a suitable ligand.
A typical application in molecular recognition involved the determination of formation constants for complexes formed between a tripodal substituted urea molecule and various saccharides.
An example of the use of supramolecular complexes in the development of chemosensors is provided by the use of transition-metal ensembles to sense for ATP.
For example, dicarboxylate anions could be encapsulated in the ellipsoidal cavity in a large macrocyclic structure containing two metal ions.
The data set used for the calculation has three components: a statement defining the nature of the chemical species that will be present, called the model of the system, details concerning the concentrations of the reagents used in the titration, and finally the experimental measurements in the form of titre and pH (or emf) pairs.
Current practice is to take absorbance or fluorescence measurements at a range of wavelengths and to fit these data simultaneously.
The most frequently used programs are: In biochemistry, formation constants of adducts may be obtained from Isothermal titration calorimetry (ITC) measurements.
All these reviews are published by IUPAC and the full text is available, free of charge, in pdf format.