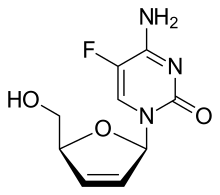
Dexelvucitabine
Dexelvucitabine is a failed experimental agent for the management of human immunodeficiency virus infection.
[1] that inhibits HIV-1 replication in vitro.
During phase II clinical trials there was some indication of a decreased mean viral load in patients with infected human immunodeficiency virus.
[2][3] On April 3, 2006, Pharmasset and Incyte, the pharmaceutical companies developing dexelvucitabine, announced the decision to cease further trials and development of the drug due to an increased incidence of grade 4 hyperlipasemia (an excess of the pancreatic enzyme lipase in the bloodstream) in a phase II trial.
This antiinfective drug article is a stub.