Discovery and development of non-nucleoside reverse-transcriptase inhibitors
Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) are antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV).
NNRTIs inhibit reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of HIV.
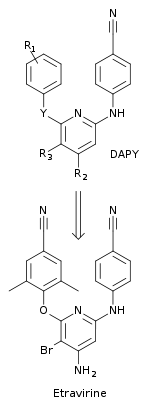
Researchers at Janssens Foundation and Tibotec discovered the first drug in this class, etravirine, which was approved by the FDA in 2008.
[7] Secondly it has ribonuclease H (Rnase H) activity as it degrades the RNA strand of RNA-DNA intermediate that forms during viral DNA synthesis.
Wing I has a functional group at one side of the ring which is capable of accepting and/or donating hydrogen bonds with the main chain of the amino acids Lys-101 and Lys-103.
On the butterfly body a hydrophobic part fills a small pocket which is mainly formed by the side chains of Lys-103, Val-106 and Val-179.
Second generation NNRTIs such as diarylpyrimidins (DAPYs), have a horseshoe-like shape with two lateral hydrophobic wings and a pyrimidine ring which is the central polar part.
[13] The NNIBP is elastic and the conformation depends on the size, specific chemical composition and binding mode of the NNRTI.
[6][7] Binding of NNRTI to HIV-1 RT makes the p66 thumb domain hyper extended because it induces rotamer conformation changes in amino acid residues Tyr-181 and Tyr-188.
[15] The global conformational change additionally destabilizes the enzyme on its nucleic acid template and reduces its ability to bind nucleotides.
[15] The development of effective anti-HIV drugs is difficult due to wide variations in nucleotide and amino acid sequences.
The discovery of the TIBO compounds led to the definition of the NNRTI class in the late 1980s[2] when they were unexpectedly found to inhibit RT.
[4][7] Both the HEPT and TIBO compounds were first to be identified as highly specific and potent HIV-1 RT inhibitors, not active against other RTs.
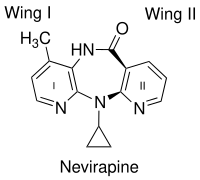
[7] After the discovery of HEPT and TIBO, compounds screening methods were used to develop BI-RG-587, the first NNRTI commonly known as nevirapine.
Like HEPT and TIBO, nevirapine blocked viral RT activity by non-competitive inhibition (with respect to dNTP binding).
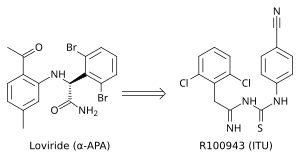
[8] Crystal structure analysis showed that the first generation NNRTIs (for example TIBO, nevirapine and α-APA) bind HIV-1 RT in a “butterfly-like” conformation.
A potent ITU compound, R100943, was obtained by an arrangement of the chemical composition of the side groups based on structure-activity relationships (SAR).
[10][17] R100943 inhibited HIV-1 and was considerably effective against a number of key NNRTI-resistant mutants like G190A mutation, which caused high-level resistance to loviride (α-APA) and nevirapine.
When compared with nevirapine and loviride which bind in the butterfly shape the ITU derivatives revealed improved activity against Tyr-181C and Tyr-188L mutants.
A structural study suggested that a potent TIBO compound could partly supplement for the effects of the Tyr-181C mutation by moving itself in the non-nucleoside inhibitor binding pocket (NNIBP) of the mutant RT.
The central part of the DATA compounds, in which the triazine ring replaced the thiourea group of ITU derivatives, is positioned between the side chains of L100 and V179.
This removed a number of torsional degrees of freedom in the central part while keeping the flexibility between the triazine ring and the wings.
R120393, a DATA analog, was designed with a chloroindole part in wing I to expand interactions with the side chain of conserved W229 of the polymerase primer grip loop.
Variability between the inhibitors could be seen when the chemical composition, size of wing I and the two linker groups connecting the rings were altered.
It is used in treatment-experienced adult patients with HIV infection that is multidrug resistant in combination with other antiretroviral drugs.
Several other NNRTIs underwent clinical development but were discontinued due to unfavourable pharmacokinetic, efficacy and/or safety factors.
[24] It is contraindicated for use with proton pump inhibitors due to the increased gastric pH causing decreased rilpivirine plasma concentrations, potentially resulting in loss of virologic response and possible resistance.
[25] A newer fixed-dose drug also combining rilpivirine with emtricitabine and tenofovir alafenamide (TAF) was approved in March 2016 under the brand name Odefsey.
[citation needed] In 2007 a new family of triazole NNRTIs was presented by researchers from the pharmaceutical company Ardea Biosciences.
[28] Lersivirine belongs to the pyrazole family and is another next generation NNRTI in clinical trials developed by the pharmaceutical company ViiV Healthcare.