Diabetic retinopathy
In at least 90% of new cases, progression to more aggressive forms of sight threatening retinopathy and maculopathy could be reduced with proper treatment and monitoring of the eyes.
[6] Around half of people with diabetic retinopathy develop swelling of the macula, called macular edema, which can begin at any time.
[5] The repeated processes of blood vessel growth, swelling, and scarring can eventually cause retinal detachment, which manifests as the sudden appearance of dark floating spots, flashes of light, or blurred vision.
[5] The next three categories: mild, moderate, and severe nonproliferative diabetic retinopathy (NPDR) describe increasing levels of damage to the retina.
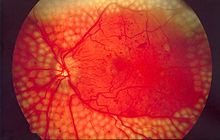
[11] Fluorescein angiography is used by retina specialists to further assess diabetic retinopathy severity and to determine sites of macular damage.
[11][12] Due to the lack of symptoms, most people with diabetic retinopathy are unaware that they have the condition until they visit an eye doctor.
For women with diabetes who become pregnant, the ADA recommends an eye examination before pregnancy, in each trimester, and for a year post partum.
[21] Poor oxygenation of tissues drives the formation of new blood vessels throughout the retina, resulting in the proliferative stage of disease.
[21] These new blood vessels tend to rupture easily, causing bleeding within the eye, scarring, and damage to the retina or macula.
[24] Several more minor risk factors also exacerbate diabetic retinopathy, namely kidney disease, abnormal blood lipids, high body mass index, and smoking.
This protection appears to be due to the elevated levels of endostatin,[27] an anti-angiogenic protein, derived from collagen XVIII.
Incidence of Retinitis Pigmentosa is observed to result in fewer and less severe microvascular lesions in both humans and mouse models.
[28] Retinitis Pigmentosa results in loss of rod receptors in the mid peripheral field, reducing the oxygen consumption that is linked with release of VEGF and growth of unwanted blood vessels in the retina.
The earliest changes leading to diabetic retinopathy include narrowing of the retinal arteries associated with reduced retinal blood flow; dysfunction of the neurons of the inner retina, followed in later stages by changes in the function of the outer retina, associated with subtle changes in visual function; dysfunction of the blood-retinal barrier, which protects the retina from many substances in the blood (including toxins and immune cells), leading to the leaking of blood constituents into the retinal neuropile.
An experimental study suggests that pericyte death is caused by blood glucose persistently activating protein kinase C and mitogen-activated protein kinase (MAPK), which, through a series of intermediates, inhibits signaling through platelet-derived growth factor receptors—signaling that supports cellular survival, proliferation, and growth.
[34][35] A genetic study showed that diabetic retinopathy shares a similar genetic predisposition with levels of glucose, low-density lipoprotein cholesterol, and systolic blood pressure,[25] indicating that glycemic control and cardiometabolic factors may be important in the development of diabetic retinopathy.
[41] Injection of anti-VEGF drugs or steroids can reduce diabetic retinopathy progression in around half of eyes treated; however, whether this results in improved vision long term is not yet known.
[43][44] Those at highest risk of vision loss – that is, with edema near the center of the macula – benefit most from eye injections of anti-VEGF therapies aflibercept, bevacizumab, or ranibizumab.
[50] For those with proliferative or severe non-proliferative diabetic retinopathy, vision loss can be prevented by treatment with panretinal laser photocoagulation.
In treating advanced diabetic retinopathy, the burns are used to destroy the abnormal new blood vessels that form in the retina.
[54] When injected in the vitreous cavity, the steroid decreases the macular edema (thickening of the retina at the macula) caused due to diabetic maculopathy, and that may result in an increase in visual acuity.
[4] There is some evidence that overall, in people with type II diabetes, fenofibrate may not make a clinically significant difference in progression of DME.
[4] For people who have type II diabetes and have overt retinopathy, there is evidence that fenofibrate may be more effective at reducing the progression of retinal damage.
Early vitrectomy is especially effective in people with insulin-dependent diabetes, who may be at greater risk of blindness from a hemorrhage into the eye.
There is evidence which suggests anti-VEGF drugs given either prior to or during vitrectomy may reduce the risk of posterior vitreous cavity haemorrhage .
[61][62] The light from the mask stops rod cells in the retina from dark adapting, which is thought to reduce their oxygen requirement, which in turn diminishes new blood vessel formation and thus prevents diabetic retinopathy.
[61] As of 2018, the results from the clinical trial showed no long-term therapeutic benefit from using the mask in diabetic retinopathy patients.
[68][69] Clinical trials are under way or are being populated in preparation for study at medical centers in Brazil, Iran and the United States.
Current trials involve using the patients' own stem cells derived from bone marrow and injected into the degenerated areas in an effort to regenerate the vascular system.
[74] Therefore, scientists have explored developing computer-aided diagnosis approaches to automate the process, which involves extracting information about the blood vessels and any abnormal patterns from the rest of the fundoscopic image and analyzing them.