Dialysis (chemistry)
In the context of life science research, the most common application of dialysis is for the removal of unwanted small molecules such as salts, reducing agents, or dyes from larger macromolecules such as proteins, DNA, or polysaccharides.
[4] From this concept dialysis can be defined as a spontaneous separation process of suspended colloidal particles from dissolved ions or molecules of small dimensions through a semi permeable membrane.
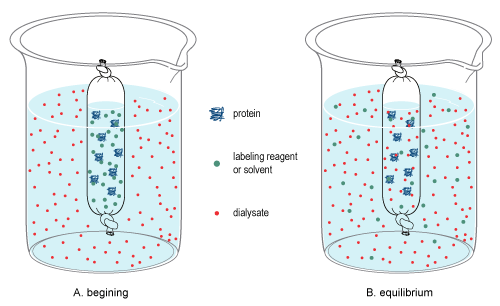
[6][7] It relies on diffusion, which is the random, thermal movement of molecules in solution (Brownian motion) that leads to the net movement of molecules from an area of higher concentration to a lower concentration until equilibrium is reached.
[6][7] For example, dialysis occurs when a sample contained in a cellulose bag and is immersed into a dialysate solution.
During dialysis, equilibrium is achieved between the sample and dialysate since only small molecules can pass the cellulose membrane, leaving only larger particles behind.
Once equilibrium is reached, the final concentration of molecules is dependent on the volumes of the solutions involved, and if the equilibrated dialysate is replaced (or exchanged) with fresh dialysate (see procedure below), diffusion will further reduce the concentration of the small molecules in the sample.
It has an increase in entropy and decrease in Gibbs free energy which means that it is thermodynamically favorable.
[8] Electrodialysis is a process of separation which uses ion-exchange membranes and an electrical potential as a driving force.
[10] Reverse electrodialysis is a technology based on membranes which gets electricity from a mixing of two water streams with different salinities.
By using the appropriate volume of dialysate and multiple exchanges of the buffer, the concentration of small contaminants within the sample can be decreased to acceptable or negligible levels.
The MWCO typically refers to the smallest average molecular mass of a standard molecule that will not effectively diffuse across the membrane during extended dialysis.
Thus, a dialysis membrane with a 10K MWCO will generally retain greater than 90% of a protein having a molecular mass of at least 10kDa.
The choice of the dialysis set up used is largely dependent on the size of the sample and the preference of the user.
Tubing provides flexibility but has increased concerns regarding handling, sealing and sample recovery.
Following the structure of the previous section, the pros and cons are discussed based on the type of dialysis used.
Another advantage is the fact that not high pressure is applied which implies that the effect fouling is not significant and consequently no chemicals are required to fight against them.
Moreover, the fouling layer is not compact which leads to a higher recovery and to a long membrane life.
In fact, it is lower in comparison with the needed in the multi effect distillation (MED) and mechanical vapour compression (MVC) processes.
The fact is that at certain voltage applied the diffusion of ions through the membrane are not linear leading to water dissociation, which would reduce the efficiency of the operation.
Finally, in the case of some products, it must be considered that electrodialysis does not remove microorganisms and organic contaminants, therefore a post-treatment is necessary.