Diamondoid
Examples include: One tetramantane isomer is the largest ever diamondoid prepared by organic synthesis using a keto-carbenoid reaction to attach cyclopentane rings.
[4] The first-ever isolation of a wide range of diamondoids from petroleum took place in the following steps:[1] a vacuum distillation above 345 °C, the equivalent atmospheric boiling point, then pyrolysis at 400 to 450 °C in order to remove all non-diamondoid compounds (diamondoids are thermodynamically very stable and will survive this pyrolysis) and then a series of high-performance liquid chromatography separation techniques.
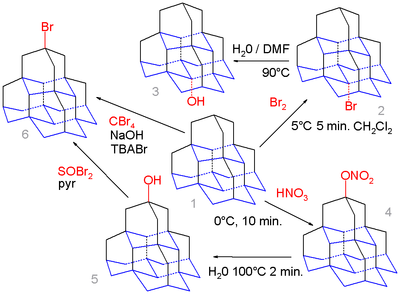
[6] The medial position (base) in this molecule (the isomer [1(2,3)4]pentamantane) is calculated to yield a more favorable carbocation than the apical position (top) and simple bromination of pentamantane 1 with bromine exclusively gives the medial bromo derivative 2 which on hydrolysis in water and DMF forms the alcohol 3.
Diamondoids are found in mature high-temperature petroleum fluids (volatile oils, condensates and wet gases).
[10][11] Diamondoids have been found to exhibit a negative electron affinity, making them potentially useful in electron-emission devices.