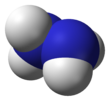
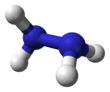
Hydrazine
[14][15][16] The nomenclature is a bi-valent form, with prefix hydr- used to indicate the presence of hydrogen atoms and suffix beginning with -az-, from azote, the French word for nitrogen.
[9] Hydrazine is also used as a long-term storable propellant on board space vehicles, such as the Dawn mission to Ceres and Vesta, and to both reduce the concentration of dissolved oxygen in and control pH of water used in large industrial boilers.
The F-16 fighter jet, Eurofighter Typhoon,[17] Space Shuttle, and U-2 spy plane use hydrazine to fuel their Emergency Start System in the event of an engine stall.
Hydrazine compounds can be effective as active ingredients in insecticides, miticides, nematicides, fungicides, antiviral agents, attractants, herbicides, or plant growth regulators.
[20] The Italian catalyst manufacturer Acta (chemical company) has proposed using hydrazine as an alternative to hydrogen in fuel cells.
By storing the hydrazine in a tank full of a double-bonded carbon-oxygen carbonyl, the fuel reacts and forms a safe solid called hydrazone.
[24] The mixture was used to power the Messerschmitt Me 163B rocket-powered fighter plane, in which the German high test peroxide T-Stoff was used as an oxidizer.
[26] In all hydrazine mono-propellant engines, the hydrazine is passed over a catalyst such as iridium metal supported by high-surface-area alumina (aluminium oxide), which causes it to decompose into ammonia (NH3), nitrogen gas (N2), and hydrogen (H2) gas according to the three following reactions:[27] The first two reactions are extremely exothermic (the catalyst chamber can reach 800 °C in a matter of milliseconds,[28]) and they produce large volumes of hot gas from a small volume of liquid,[29] making hydrazine a fairly efficient thruster propellant with a vacuum specific impulse of about 220 seconds.
[32] There are ongoing efforts in the aerospace industry to find a replacement for hydrazine, given its potential ban across the European Union.
[33][34][35] Promising alternatives include nitrous oxide-based propellant combinations, with development being led by commercial companies Dawn Aerospace, Impulse Space,[36] and Launcher.
[37] The first nitrous oxide-based system ever flown in space was by D-Orbit onboard their ION Satellite Carrier in 2021, using six Dawn Aerospace B20 thrusters.
[40] Hydrazine exposure can cause skin irritation/contact dermatitis and burning, irritation to the eyes/nose/throat, nausea/vomiting, shortness of breath, pulmonary edema, headache, dizziness, central nervous system depression, lethargy, temporary blindness, seizures and coma.
The American Conference of Governmental Industrial Hygienists (ACGIH) grades hydrazine as "A3—confirmed animal carcinogen with unknown relevance to humans".
The U.S. Environmental Protection Agency (EPA) grades it as "B2—a probable human carcinogen based on animal study evidence".
[44] Based on cohort and cross-sectional studies of occupational hydrazine exposure, a committee from the National Academies of Sciences, Engineering and Medicine concluded that there is suggestive evidence of an association between hydrazine exposure and lung cancer, with insufficient evidence of association with cancer at other sites.
The genotoxic mechanism the committee cited references hydrazine's reaction with endogenous formaldehyde and formation of a DNA-methylating agent.
[48] A complete surveillance programme for hydrazine should include systematic analysis of biologic monitoring, medical screening and morbidity/mortality information.
Pre-placement and periodic medical screening should be conducted with specific focus on potential effects of hydrazine upon functioning of the eyes, skin, liver, kidneys, hematopoietic, nervous and respiratory systems.
[40] Common controls used for hydrazine include process enclosure, local exhaust ventilation and personal protective equipment (PPE).
[40] Guidelines for hydrazine PPE include non-permeable gloves and clothing, indirect-vent splash resistant goggles, face shield and in some cases a respirator.
Specific guidance for exposure response includes mandatory emergency shower and eyewash stations and a process for decontaminating protective clothing.
These structural properties resemble those of gaseous hydrogen peroxide, which adopts a "skewed" anticlinal conformation, and also experiences a strong rotational barrier.
[9] The net reaction is:[51] In this route, the ketone and ammonia first condense to give the imine, which is oxidised by hydrogen peroxide to the oxaziridine, a three-membered ring containing carbon, oxygen, and nitrogen.
[52] The Olin Raschig process, first announced in 1907, produces hydrazine from sodium hypochlorite (the active ingredient in many bleaches) and ammonia without the use of a ketone catalyst.
It also directly reduces salts of less active metals (e.g., bismuth, arsenic, copper, mercury, silver, lead, platinum, and palladium) to the element.
Some colour photographic processes also use a weak solution of hydrazine as a stabilising wash, as it scavenges dye coupler and unreacted silver halides.
[62] Hydrazines are part of many organic syntheses, often those of practical significance in pharmaceuticals (see applications section), as well as in textile dyes and in photography.
Being bifunctional, with two amines, hydrazine is a key building block for the preparation of many heterocyclic compounds via condensation with a range of difunctional electrophiles.
[68] The false morel produces the poison gyromitrin which is an organic derivative of hydrazine that is converted to monomethylhydrazine by metabolic processes.
[69][70] In the fictional book The Martian (also adapted to a feature film) the titular character uses an iridium catalyst to separate hydrogen gas from surplus hydrazine fuel, which he then burns to generate water for survival.