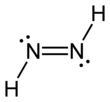
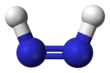
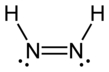Diimide
A traditional route to diimide involves oxidation of hydrazine with hydrogen peroxide or air.
Even at low temperatures, the more stable trans isomer rapidly undergoes various disproportionation reactions, primarily forming hydrazine and nitrogen gas:[5] Because of this competing decomposition reaction, reductions with diimide typically require a large excess of the precursor reagent.
Although the method is cumbersome, the use of diimide avoids the need for high pressures or hydrogen gas and metal catalysts, which can be expensive.
Thus, peroxides, alkyl halides, and thiols are tolerated by diimide, but these same groups would typically be degraded by metal catalysts.
[4] The dicationic form, H−N+≡N+−H (diazynediium, diprotonated dinitrogen), is calculated to have the strongest known chemical bond.