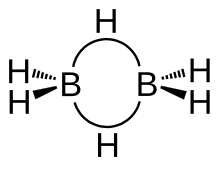
Diborane
Aluminium forms a polymeric hydride, (AlH3)n; although unstable, Al2H6 has been isolated in solid hydrogen and is isostructural with diborane.
Similarly, oxidation of borohydride salts has been demonstrated and remains convenient for small-scale preparations.
For example, using iodine as an oxidizer:[13] Another small-scale synthesis uses potassium borohydride and phosphoric acid as starting materials.
Methanol for example give hydrogen and trimethylborate:[17] One dominating reaction pattern involves formation of adducts with Lewis bases.
This reaction pattern is rather general and the resulting alkyl borates can be readily derivatized, e.g. to alcohols.
Although early work on hydroboration relied on diborane, it has been replaced by borane dimethylsulfide, which is more safely handled.
[18][19] Although this pyrolysis route is rarely employed, it ushered in a large research theme of borane cluster chemistry.
[20] Diborane is used as a reducing agent roughly complementary to the reactivity of lithium aluminium hydride.
The compound readily reduces carboxylic acids to the corresponding alcohols, whereas ketones react only sluggishly.
[21] Electron diffraction measurements by S. H. Bauer initially appeared to support his proposed structure.
[28] William Nunn Lipscomb Jr. further confirmed the molecular structure of boranes using X-ray crystallography in the 1950s and developed theories to explain their bonding.
Later, he applied the same methods to related problems, including the structure of carboranes, on which he directed the research of future 1981 Nobel Prize winner Roald Hoffmann.
The 1976 Nobel Prize in Chemistry was awarded to Lipscomb "for his studies on the structure of boranes illuminating problems of chemical bonding".
[29] Traditionally, diborane has often been described as electron-deficient, because the 12 valence electrons can only form 6 conventional 2-centre 2-electron bonds, which are insufficient to join all 8 atoms.
[35][36][37] Diborane has been investigated as a precursor to metal boride films[38] and for the p-doping of silicon semiconductors.