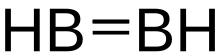Diborane(2)
Diborane(2) is a highly reactive molecule that rapidly decomposes, making it a challenge to study experimentally under ambient conditions.
To observe diborane(2) experimentally, high-vacuum and low temperature conditions using matrix isolation techniques are required, such as trapping the molecule in inert matrices like neon or argon.
The first experiment that lead to the synthesis of diborane(2) was via pulsed laser ablation of boron in a mixed hydrogen-argon gas atmosphere.
Following this experiment, there have been other methods of diborane(2) preparation by decomposition of gaseous B2H6 via photoionization, electron bombardment, X-irradiation, high-temperature reactions, and pulsed laser vaporization.
Theoretical data found that the molecule has a 3Σ-g ground state conformation, indicating a particular orientation with threefold rotational symmetry and a vertical mirror plane.
This theoretical data was confirmed experimentally, which found the molecule to be linear with a triplet ground state, as revealed by electron paramagnetic resonance.