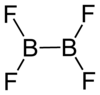
Diboron tetrafluoride
A colorless gas, the compound has a halflife of days at room temperature.
It is the most stable of the diboron tetrahalides,[1] and does not appreciably decompose under standard conditions.
[2] Diboron tetrafluoride is a planar molecule with a B-B bond distance of 172 pm.
[1] Although it is electron-deficient, the unsaturated boron centers are stabilized by pi-bonding with the terminal fluoride ligands.
Diboron tetrafluoride can be formed by treating boron monofluoride with boron trifluoride at low temperatures, taking care not to form higher polymers.