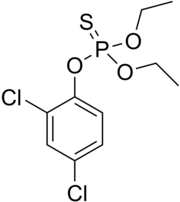
Dichlofenthion
Dichlofenthion (IUPAC name: O-(2,4-dichlorophenyl) O,O-diethyl phosphorothioate)[citation needed] is a fat-soluble organophosphorus compound primarily used in agricultural practices as a pesticide and nematicide to control a variety of insect pests.
Organophosphates as a class were initially developed in the early 20th century, with some of the earliest compounds being synthesized as potential chemical warfare agents during and after World War I.
However, their potent action on the nervous system quickly led to their adoption in agriculture to control pests that affect crops.
Dichlofenthion emerged in the mid-20th century as part of the search for more effective and selective insecticides that could provide better crop protection with reduced environmental and health impacts.
Source:[1] The molecular structure of dichlofenthion includes a diethyl phosphorothioate group (C4H11O3PS) attached to a dichlorophenyl ring.
The synthesis of dichlofenthion involves the reaction of 2,4-dichlophenol with diethyl thiophosphoryl chloride, which requires careful control of conditions to ensure the formation of the desired product and minimize by-products.
[3] A-esterase are located in plasma and hepatic endoplasmic reticulum and can hydrolyse organophosphorus compounds by splitting the anhydride, P-F, P-CN, or ester bond.
However, it is notable that the biotransformation pathways of dichlofenthion can vary based on species, individual genetic makeup, and environmental factors due to the presence and absence of different enzymes.
The detailed uses, availability, and efficacy of dichlofenthion involve several aspects, including its application on crops, regulatory status, and effectiveness against pests.
Where it is registered for use, dichlofenthion can be found in various formulations mentioned above, including emulsifiable concentrates and granules, designed to suit different application needs and crop types.
Therefore, integrated pest management (IPM) practices, including the rotation of insecticides with different modes of action, are recommended to preserve the efficacy of products like dichlofenthion.
The detailed mechanism of action of dichlofenthion, an organophosphate insecticide, involves the inhibition of the enzyme acetylcholinesterase (AChE),[10] which plays a critical role in nerve signal transmission.
[15] Dichlofenthion exhibits delayed onset symptoms, meaning there is a significant time lapse between ingestion and the appearance of noticeable effects.
Despite this delayed onset, dichlofenthion poisoning can cause severe and life-threatening symptoms, emphasizing the importance of prompt medical attention.
The goal of administering atropine is to prevent bradycardia, maintain blood pressure, clear lungs and dry skin.
[16] Pralidoxime is an oxime compound that can reactivate acetylcholinesterase inhibited by organophosphate pesticides like dichlofenthion, if administered early.
If exposure to dichlofenthion occurs through ingestion or dermal contact, decontamination procedures such as rinsing the skin or eyes with water and removing contaminated clothing should be performed promptly to minimize further absorption of the pesticide.
[15] It is essential to seek medical attention immediately in cases of suspected dichlofenthion poisoning to ensure appropriate treatment and prevent potentially life-threatening complications.
Additionally, proper handling and application practices should be followed to minimize the risk of exposure to organophosphate pesticides like dichlofenthion.
[20] NOAEL: 0.75 mg/kg (rat) This refers to the lowest dose of a physical agent or chemical substance that causes harmful effects.
In areas where dichlofenthion has been used or is still in use, individuals involved in agricultural activities, including farmers, farmworkers, and pesticide applicators, may be at risk of exposure.
Proper handling, application, and safety precautions are essential to minimize the risks of exposure to dichlofenthion and other pesticides in agricultural settings.
The effectiveness of dichlofenthion must be balanced with considerations of its environmental persistence, potential for bioaccumulation, and toxicity to non-target organisms, including humans.
Persistence and degradation: provides data about how long does a certain chemical remain active in the environment; this data includes half-life, in soil and water, and its degradation, which happens through processes such as photolysis, hydrolysis, microbial action... Dichlofenthion persistence in the environment can pose risks to wildlife and contaminate water sources.
Human acute organophosphorus poisoning can result from occupational, accidental, criminal, or intentional exposure (i.e. suicidal ingestion).
Acute muscarinic effects on the heart can be life-threatening,[11] including bradycardia, hypotension, and cardiac conduction abnormalities due to heightened vagal tone.
The nicotinic effects of dichlorofenthion predominantly target neuromuscular junctions, triggering a range of muscular and neurological symptoms.
Additionally, dichlorofenthion's interaction with nicotinic receptors in the central nervous system can induce neurological manifestations such as headache, dizziness, and confusion.
[11] In severe cases, dichlorofenthion poisoning may progress to seizures and coma, highlighting its profound impact on both muscular and neurological functions.
This parameter measures whether a compound could potentially cause adverse effects on the fertility and sexual functioning of adults as well as their offspring.