Diethylaluminium chloride
Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound.
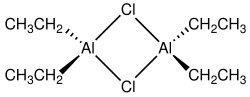
Although often given the chemical formula (C2H5)2AlCl, it exists as a dimer, [(C2H5)2AlCl]2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins.
The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents.
[3][4] In contrast, triethylaluminium and trimethylaluminium feature bridging alkyl groups and these compounds violate the octet rule.
Diethylaluminium chloride can be produced from ethylaluminium sesquichloride, (C2H5)3Al2Cl3, by reduction with sodium:[5] It is also obtained from the reaction of triethylaluminium with hydrochloric acid: Reproportionation reactions can also be used: Diethylaluminium chloride and other organoaluminium compounds are used in combination with transition metal compounds as Ziegler–Natta catalysts for the polymerization of various alkenes.