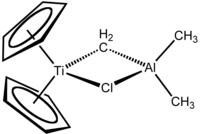
Tebbe's reagent
Tebbe's reagent itself does not react with carbonyl compounds, but must first be treated with a mild Lewis base, such as pyridine, which generates the active Schrock carbene.
Also analogous to the Wittig reagent, the reactivity appears to be driven by the high oxophilicity of Ti(IV).
The Schrock carbene (1) reacts with carbonyl compounds (2) to give a postulated oxatitanacyclobutane intermediate (3).
[8] [9][10] This conversion can also be effected using the Wittig reaction, although the Tebbe reagent is more efficient especially for sterically encumbered carbonyls.
For this reason, the Tebbe reagent has found applications in reactions of sugars where maintenance of stereochemistry can be critical.
[11] The Tebbe reagent reacts with acid chlorides to form titanium enolates by replacing Cl−.