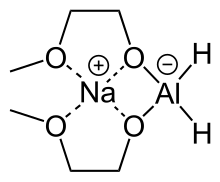
Sodium bis(2-methoxyethoxy)aluminium hydride
The compound features a tetrahedral aluminium center attached to two hydride and two alkoxide groups, the latter derived from 2-methoxyethanol.
At low temperatures (below -60°C), the solution solidifies to a glassy pulverizable substance with no sharp melting point.
It readily converts epoxides, aldehydes, ketones, carboxylic acids, esters, acyl halides, and anhydrides to the corresponding alcohols.
SMEAH exhibits similar reducing effects, but does not have the inconvenient pyrophoric nature, short shelf-life, or limited solubility of LAH.
[1] SMEAH in toluene under reflux has been used to reduce aliphatic p-toluenesulfonamides (TsNR2) to the corresponding free amines and is one of the few reagents that can carry out this challenging reduction in general settings.