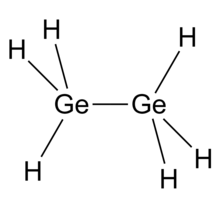
Digermane
[3] Many of the properties of digermane and trigermane GeH3GeH2GeH3 were determined in the following decade using electron diffraction studies.
Although the major product is germane, a quantifiable amount of digermane is produced in addition to traces of trigermane.
[6] The reactions of digermane exhibit some differences between analogous compounds of the Group 14 elements carbon and silicon.
As seen in the last reaction of the mechanism above, pyrolysis of digermane may induce polymerization of the GeH2 group, where GeH3 acts as a chain propagator and molecular hydrogen gas is released.
These trifluoromethylthio (−S−CF3) and trifluoromethylseleno (−Se−CF3) derivatives possess a markedly higher thermal stability than digermane itself.