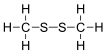Dimethyl disulfide
Dimethyl disulfide (DMDS) is an organic chemical compound with the molecular formula CH3SSCH3.
It is a flammable liquid with an unpleasant, garlic-like odor resembling that of "leaking gas".
[9][10] DMDS is used to prepare catalysts for hydrodesulfurization, because of its high sulfur content and low decomposition temperature.
[12] DMDS also works as an effective product for operators in the petrochemicals industry who must protect their steam-cracking coils against the formation of coke and carbon monoxide.
DMDS and chlorine are reacted with borontrifluoride phenoxide to produce 4-(methylthio)phenol.