Diol
Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol HO−(CH2)4−OH and propylene-1,3-diol, or beta propylene glycol, HO−CH2−CH2−CH2−OH.
The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2.
Another example is propane-1,2-diol, or alpha propylene glycol, HO−CH2−CH(OH)−CH3, used in the food and medicine industry, as well as a relatively non-poisonous antifreeze product.
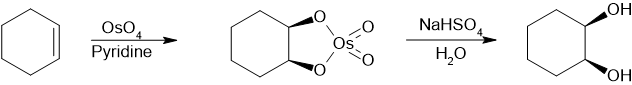
An example in the synthesis of trans-cyclohexanediol[6] or by microreactor:[7] For academic research and pharmaceutical areas, vicinal diols are often produced from the oxidation of alkenes, usually with dilute acidic potassium permanganate or Osmium tetroxide.
1,3-Diols are described as syn or anti depending on the relative stereochemistries of the carbon atoms bearing the hydroxyl functional groups.
Diols where the hydroxyl groups are separated by several carbon centers are generally prepared by hydrogenation of diesters of the corresponding dicarboxylic acids: 1,4-butanediol, 1,5-pentanediol, 1,6-hexanediol, and 1,10-decanediol [es] are important precursors to polyurethanes.
[11] From the industrial perspective, the dominant reactions of the diols is in the production of polyurethanes and alkyd resins.
[12] Diols such as ethylene glycol are used as co-monomers in polymerization reactions forming polymers including some polyesters and polyurethanes.
Then, followed by intramolecular nucleophilic substitution, the second hydroxyl group attacks the electron deficient carbon.
In glycol cleavage, the C−C bond in a vicinal diol is cleaved with formation of ketone or aldehyde functional groups.
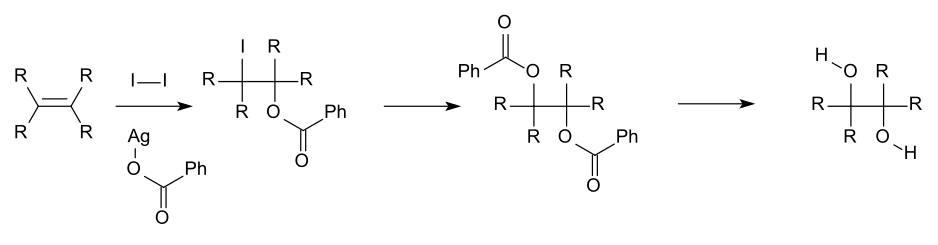
In general, organic geminal diols readily dehydrate to form a carbonyl group.